Abstract
Background and Aims. Minimal residual disease (MRD) detection is a validated outcome predictor in mantle cell lymphoma (MCL). Recently, the clinical relevance of repeated MRD monitoring has been described in a prospective clinical trial [Ferrero, Blood 2022]. Nonetheless, complex patterns of MRD kinetics over time and availability of results obtained by heterogeneous tissues (bone marrow, BM, vs peripheral blood, PB) might generate substantial interpretation issues and hamper an easy-to-use application of this predictive biomarker. To overcome the limitation of empirical analysis we tested CONNECTOR, a novel automated computational framework to facilitate the interpretation of MRD kinetics and stratify patients in risk classes based on a solid, algorithm-derived, classification.
Patients and methods. ASO RQ-PCR MRD data already generated from BM and PB samples in the FIL MCL0208 trial [Ferrero, Blood 2022], offering first-line high dose chemoimmunotherapy and autologous transplantation (ASCT) to youngers MCL patients, were employed. CONNECTOR (https://qbioturin.github.io/connector/) is a data-driven framework based on Functional Data Analysis for analyzing longitudinal high-dimensional data in a straightforward and revealing way. CONNECTOR allows the aggregation of time-series data through an unsupervised approach in informative clusters. Moreover, we developed a classification approach to predict the risk class for new MRD data. The new MRD curves were allocated to existing risk clusters defined on a training set of MRD data.
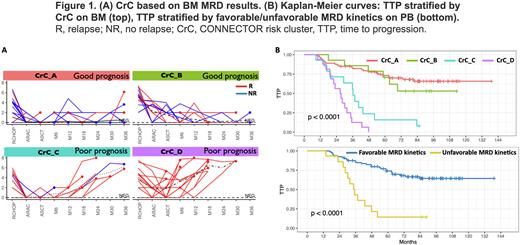
Results. Without knowledge of any clinical or outcome data, CONNECTOR was run on 117 patients characterized by at least 3 MRD timepoints in BM (median 6, 3-9) and separately on 95 patients with at least 4 MRD timepoints in PB (median 7, 4-9). Starting from BM MRD data, CONNECTOR identified four patient risk Clusters (CrC), based on MRD kinetics, Figure1A. CrC_A comprised 73 patients with an overall quick and stable MRD negativization; CrC_B accounted for 14 patients characterized by later MRD negativization, alternating MRD results, or low-level MRD persistence; the 14 patients in CrC_C were always characterized by early MRD negativization followed by late MRD reappearance; CrC_D was composed of 16 patients with stable MRD positivity or only transient MRD negativization followed by early MRD reappearance. The different trends of MRD kinetics dissected by CONNECTOR fairly predicted patients’ outcomes: median TTP was not reached for CrC_A and B, 36 months for CrC_C and 27 months for CrC_D, respectively (p<0.0001), Figure1B. Similar data were generated when CONNECTOR was run on MRD results from PB samples: CrC allocation of BM vs PB was concordant for 89% of patients and survival analysis on PB results confirmed patients stratification in two groups: favorable MRD kinetics (CrC_A and B, median TTP not reached) vs unfavorable MRD kinetics (CrC_C and D, median TTP 35 months, p<0.0001), as depicted in Figure1B. Finally, we tested the performance of pooling together BM and PB results, to increase the number of evaluable patients (irrespective of eventual missing BM samples). Only the BM point at 12 months after ASCT was considered, together with the PB MRD results of all the available time points. CONNECTOR was used to verify if this mixed MRD pattern was able to correctly reclassify patients in the same CrC. Actually, 89 out of 95 patients (94%) were correctly reclassified (p_mean=0.96, p_sd= 0.07).
Discussion. CONNECTOR allowed unsupervised identification of patients clusters with distinct MRD kinetics and highly significant different clinical outcome. Such clusterization proved effective using BM, PB and mixed tissues, as well. Validation of CONNECTOR performance in large, independent prospective trials is currently ongoing.
Disclosures
Ferrero:Gentili: Speakers Bureau; Gilead: Research Funding; Morphosys: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jannsen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Clinigen: Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Speakers Bureau. Cavallo:Amgen: Other: Expenses for EHA virtual meeting; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Expenses for Ash meeting; Takeda: Other: Expenses for ICML virtual meeting; Servier: Speakers Bureau. Balzarotti:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astrazeneca: Honoraria; Takeda: Honoraria; Servier: Membership on an entity's Board of Directors or advisory committees; Gentili: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Zilioli:Gentili: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Roche: Consultancy; MSD: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: travel expenses, Speakers Bureau. Arcaini:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal